1.2 Distal regulation by enhancers
Transcription factors regulate transcription of genes by binding to genomic DNA. However, these regulatory binding events do not occur only at proximal promoters of genes. In contrast, many transcription factors regulate genes by binding to distal regulatory elements, such as enhancers (Spitz and Furlong 2012). Physical interactions between enhancers their target gene promoter is facilitated by chromatin looping and propagated by proteins such as the mediator complex (Andrey and Mundlos 2017).
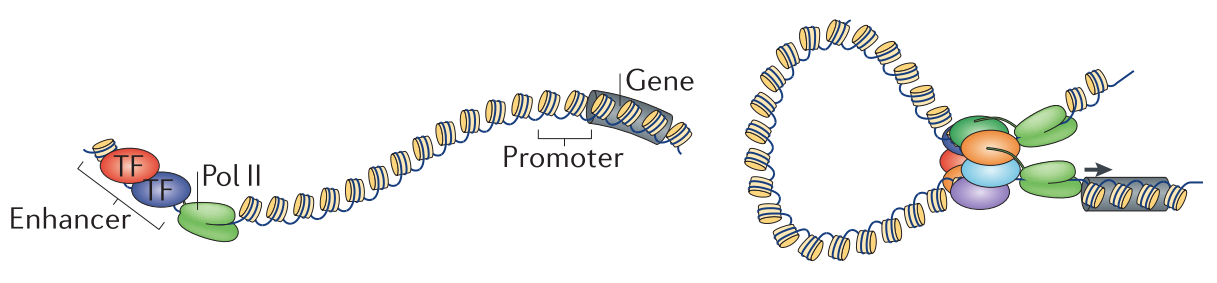
Figure 1.1: Enhancer-promoter interactions by chromatin looping. Eukaryotic genes are regulated by binding of transcription factors (TF) to DNA. Transcription factor binding events occur at proximal promoters of genes and distal regulatory elements, such as enhancers (left). Chromatin looping facilitates physical interactions of enhancers with promoters to enable distal-regulation of transcription. Figure adapted from (Pombo and Dillon 2015).
Enhancers were originally defined as genomic regions that enhance the expression of a reporter gene when placed experimentally in front of a minimal promoter. (Banerji et al. 1981; Shlyueva et al. 2014). Enhancer activity can also be detected genome-wide by specific genomic experimental approaches. Thereby, patterns of open chromatin are identified using DNase-seq (Song and Crawford 2010) or ATAC-seq (Buenrostro et al. 2013). Active enhancers show short bidirectional transcription, which can be identified using CAGE (Andersson et al. 2014). Enhancer associate with specific patterns of posttranslational histone modifications, such as H3K27ac and H3K4me1. These epigenetic marks, as well as TF binding events, can be detected genome-wide by chromatin immunoprecipitation coupled with sequencing (ChIP-seq) experiments (Creyghton et al. 2010).
Single enhancers often regulate multiple genes. Similarly, individual genes can be regulated by multiple enhancers. Thereby, additive effects of multiple enhancers often archive complex regulation of developmental genes. For example, the \(\alpha\)-globin gene locus is controlled by multiple enhancers, whereby each act independently and in an additive fashion without evidence of synergistic effects (Hay et al. 2016). Also, the Indian hedgehog (Ihh) locus is regulated by multiple enhancers with individual combinations of tissue specificities that function in an additive manner (Will et al. 2017). Variation of the copy number of enhancers by experimental perturbations correlates with expression strength. For example, a significant reduction of the expression of the oncogene PIMI could not be achieved by perturbing a single enhancer, but only by combinatorial repression of several weak enhancers (Xie et al. 2017).
While large-scale efforts provide genome-wide maps of enhancers and their activity in different human tissues (Andersson et al. 2014), it is challenging to interpret the outcome of enhancer activity to gene expression. Enhancers can be located several hundred kb apart from the promoter of regulated genes. Genome-wide studies suggest that the majority of enhancers do not regulate the next gene (Sanyal et al. 2012). Therefore, it is critical to understand long-range genome folding to illuminate the re-wiring between enhancers and regulated genes.